AUGUST 27, 2018. BY STAN GRANT.

Figure 1: An old adage: wine begins in the vineyard.
You probably have heard the phrase ‘wine begins in the vineyard’ (Figure 1). It is another way of saying that many of the compounds contributing to the color, flavor, aroma, and mouthfeel of wines are already present in the harvested grapes upon arrival at the winery.
A number of winemakers also believe some problems they encounter in the winery originate in the vineyard. They are correct in some instances, but in others they are not. To have meaningful discussions with winemakers, we need to be conversant about winery problems that can originate in vineyards. Three such problems are the topics of this article.
High pH and Potassium
In California, high grape pH is sometimes a winemaker complaint. pH is a scale used to measure the amount of active acidity or free hydrogen (H+) in solutions, such as grape musts and wines. There is a marked inverse (logarithmic) relationship between pH values and the concentration of active hydrogen, such that the quantity of hydrogen decreases significantly as the pH increases modestly.
The chemical and microbiological stability of wines also decrease as the pH increases above a value of about 3.5. Due to these instabilities, wines with high pH tend to taste flat or flabby, appear dull in color, and are prone to microbial spoilage. All of these, of course, are serious concerns for winemakers.
Potassium (K+) is part of the fruit pH story, but to understand its role we need to first consider some other aspects of potassium in vineyards. In the majority of California vineyard soils, available potassium levels are low (soluble K ≤ 15 ppm and extractable K ≤ 120 ppm). Further, potassium movement towards roots is slow, occurring mainly through random motions in the thin films of water adhering to soil particles.
Upon reaching roots, potassium is selectively taken up through an energy consuming process and its loading into vascular tissues is similarly regulated. At the same time, potassium is the most abundant positively charged ion in the above-ground vine and in grape berries (≈ 5 lb/ton). Due to the combination of limited supply, regulated uptake, and high demand, potassium in grapevines is commonly sub-optimal.
Once inside of grapevines, potassium is highly mobile, often moving towards growing organs and frequently redistributed from older to younger tissues. Within cells, potassium serves several vital roles, one of which is contributing to energy (osmotic) gradients that draw water and dissolved molecules, such as sugars, into berry cells. As a consequence, potassium deficiencies impede fruit maturation.
Another role of potassium is the neutralization of negatively charged ions, including organic acid anions like malate and tartrate. In this role, potassium can serve as a chemical substitute for hydrogen in grape berries and wines. In so doing, high fruit potassium can lead to high fruit pH.
Winemakers sometimes blame potassium fertilization for high wine pH. While it is true that excessive potassium fertilization can cause fruit pH to increase, this seldom occurs due to low potassium supplies in soils, the regulation of potassium uptake, the considerable expense of fertilization, and the cautiousness of most wine grape growers (Table 1).
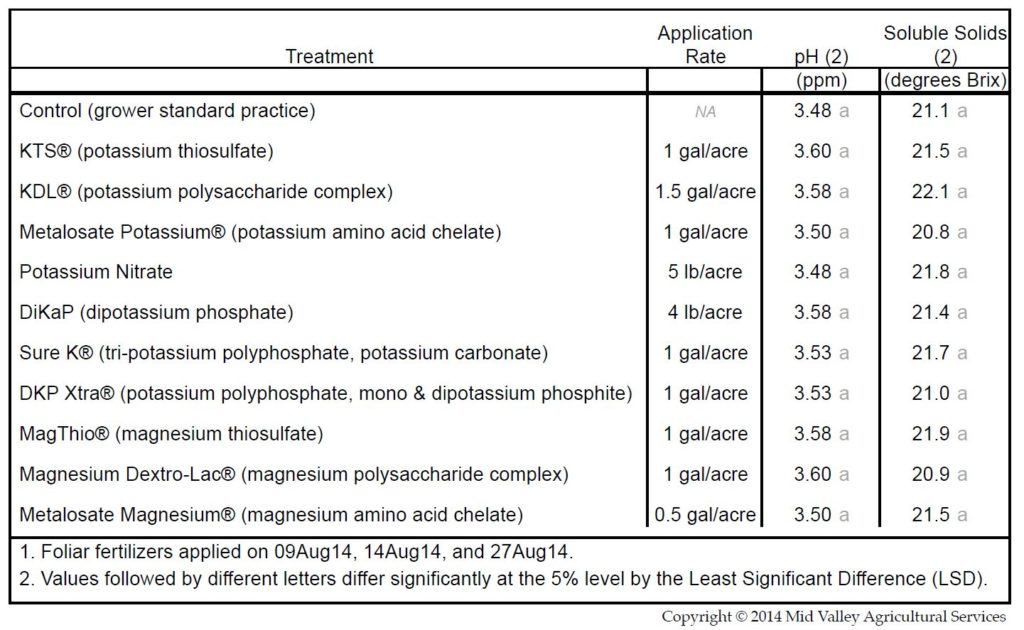
Table 1: Three foliar applications of potassium and magnesium fertilizers during ripening had a negligle effect on Malbec fruit pH. (Source: Mid Valley Agricultural Services. Used with permission.) Click HERE to enlarge table.
Rather, potassium commonly becomes elevated and pH rises in fruit due to other factors. These may include abundant soil moisture during ripening, immoderate growth vigor and excessive canopy shading, under-cropping, warm nights, inhibited photosynthesis due to stresses, and over-ripening. These are essential points to share with winemakers, should high fruit pH become a topic of conversation.
Volatile Acidity

Figure 2: Sour rot may be a source of Acetobacter and volatle acidity in wines. (Progressive Viticulture©)
High volatile acidity (VA) is another winemaker concern. In wines, the volatile acids include acetic, formic, propionic, and trace amounts of lactic and succinic acids. Of these, the most prevalent by far is acetic acid. When acetic acid is present at sensible quantities, it imparts the sharp aroma and flavor associated with vinegar.
Acetic acid is a normal byproduct of yeasts during fermentation and the small quantities present in most wines (≈ 0.2 g/L) have no adverse affects on taste. It is when concentrations are substantially higher (approaching 1.5 g/L) that a vinegary flavor becomes detectable in wines.
Such high concentrations may be due to the activities of certain group of bacteria (Acetobacter) that cause a reaction between alcohol and air during fermentation. They may also be due to the activities of Acetobacter in the harvested fruit if it contains significant quantities of bunch rot (Figure 2).
Obviously, competent vineyard and winery practices control both of these causes of high VA. Sometimes, however, VA may be high even under sound vineyard and winery management due to another cause – overripe fruit (Figure 3).

Figure 3: An overripe cluster: another potential source of Acetobacter and volatile acidity in wines. (Progressive Viticulture©)
When fruit hangs on vines beyond full ripeness it desiccates; its tissues senesce and they begin to breakdown. At the same time, its susceptibility to secondary pathogens, including Acetobacter, increases. At the winery, high sugar musts of crushed overripe fruit often slowly ferment and may become stuck. Thus, VA is likely higher than normal for wines made from overripe fruit due to both berry condition and fermentation rate. To be fair, all of the possible sources of high VA ought to be considered in any discussions you may have with your winemaker about this issue.
Yeast Assimilable Nitrogen
There is another cause of slow or stuck fermentations, but in this case there are other effects. These include rotten egg aromas due to elevated hydrogen sulfide (H2S). Winemakers sometimes blame fungicidal sulfur applications for rotten egg aromas, but research has shown sulfur applications will have only a very slight impact on sulfur residues on grapes if the last application occurred five weeks before harvest.
There is, however, a consistent relationship between elevated hydrogen sulfide characteristics and low levels of yeast assimilable nitrogen (YAN). YAN is the sum of alpha-amino acids and ammonium-nitrogen (NH4-N). These are the forms of nitrogen in grape musts most easily consumed by yeasts during fermentation. A generally accepted minimum YAN requirement is 140 ppm, but it may be higher depending on the yeast strain.
In the Northern Interior of California, low YAN wine grapes are most common from vineyards on older, former rangeland soils at the base of the Sierra Nevada. These soils were inherently low in fertility, which further declined following conversion to vineyard.
Fortunately, grape growers can increase fruit YAN through appropriate nitrogen fertilization practices applied from early fruit set through the beginning of ripening. Fertigation with liquid calcium nitrate (CN-9) increases fruit YAN under some circumstances, but effective rates (≥ 6 to 10 gallons) per acre may interfere with efforts to control shoot growth. Alternatively, two or three high-rate foliar (≈ 20 lb/acre) applications of low biuret urea increases fruit YAN with little or no impact on shoot elongation (Figure 4). To be effective, vines need to contain adequate levels of other mineral nutrients, including sulfur, potassium, iron, manganese, copper, boron, and molybdenum. (Caution: urea applications rates higher than 20 to 25 lb/acre may be phototoxic to foliar tissues).
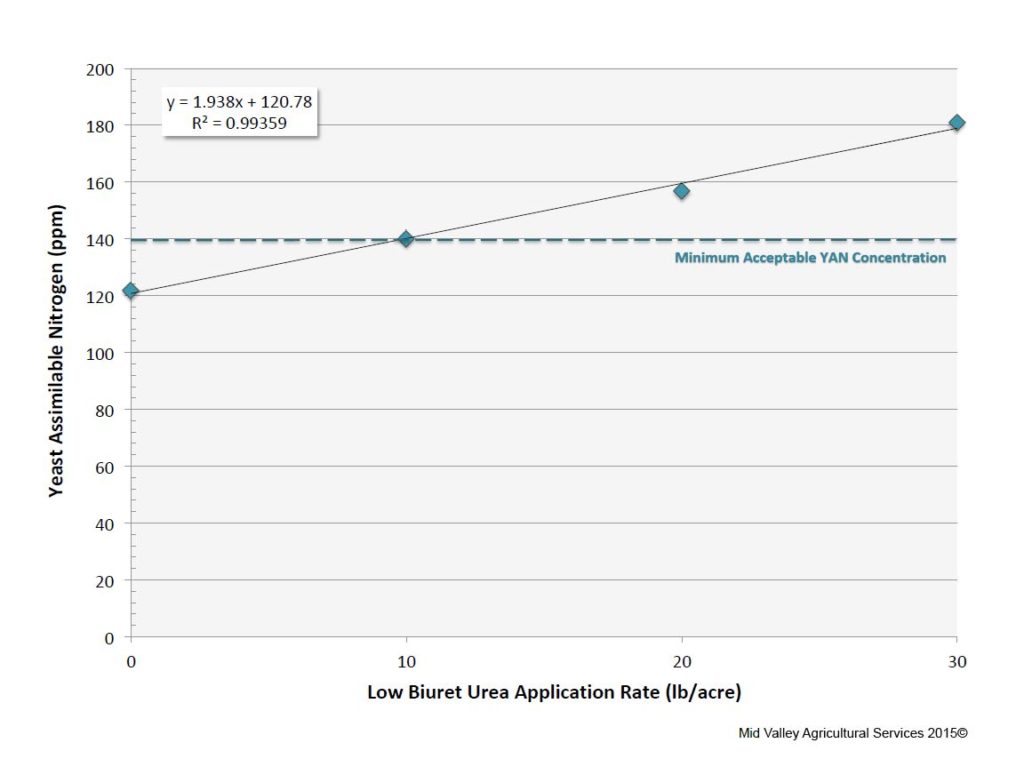
Figure 4: Merlot yeast assimilable nitrogen at harvest as a function of rate of low biuret urea applied twice to the foliage post bloom. (Source: Mid Valley Agricultural Services. Used with permission.) Click HERE to enlarge image.
A version of this article was originally published in the Mid Valley Agricultural Services September, 2010 newsletter and was updated for this blog post.
Further Reading
Bell, AA; Ough, CS; Kliewer, WM. Effects on must and wine composition, rates of fermentation, and wine quality of nitrogen fertilization of Vitis vinifera var. Thompson Seedless grapevines. Am. J. Enol. Vitic. 30, 124-129. 1979.
Boulton, R. The relationship between total acidity, titratable acidity, and pH in grape tissue. Vitis. 19, 113-120. 1980.
Boulton, R. The relationship between total acidity, titratable acidity, and pH in wine. Am. J. Enol. Vitic. 31, 76-80. 1980.
Butzke, CE; Boulton, R. Acidity, pH, and potassium for grape growers. Practical Winery and Vineyard. 10-16. Sep/Oct 1997.
Butzke, CE. Survey of yeast assimilable nitrogen status of musts from California, Oregon, and Washington. Am. J. Enol. Vitic. 49, 220224. 1998.
Dukes, B; Goldspink, B; Elliot, J; Frayne, R. Time of nitrogenous fertilization can reduce fermentation time and improve wine quality. In International Symposium on Nitrogen in Grapes and Wine. Rantz, JM (Ed.). pp. 249-254. Am. Soc. Enol. Vitic., Davis. 1990.
Hale, CR. Relationship between potassium and the malate and tartrate contents of grape berries. Vitis. 16, 9-19. 1977.
Iland, PG. Grape berry ripening: the potassium story. Australian Grapegrower and Winemaker. 22-24. Jan, 1988.
Kudo, M; Vagloli, P; Bisson, L. Imbalance of pH and potassium concentration as a cause of stuck fermentations. Am. J. Enol. Vitic. 49, 295-301. 1998.
Kunkee, RE. Relationship between nitrogen content of the must and sluggish fermentation. In International Symposium on Nitrogen in Grapes and Wine. Rantz, JM (Ed.). pp. 148-155. Am. Soc. Enol. Vitic., Davis. 1990.
Marschner, H. Mineral nutrition of higher plants. 2nd Ed. Academic Press, London. 1995.
Morris, JR; Cawthon, DL; Fleming, JW. Effects of high rates of potassium fertilization on raw product quality and changes in pH and acidity during storage of Concord grape juice. Am. J. Enol. Vitic. 31, 323-328. 1980.
Morris, JR; Sims, CA; Cawthon, DL. Effects of excessive potassium levels on pH, acidity, and color of fresh and stored grape juice. Am. J. Enol. Vitic. 34, 35-39. 1983.
Mpelasoka, BS; Schachtman, DP; Treeby, MT; Thomas, MR. A review of potassium nutrition in grapevines with a special emphasis on berry accumulation. Australian Journal of Grape and Wine Research. 9, 154-168. 2003.
Neilsen, GH; Neilsen, D; Bowen, P, Bogdanoff, C; Usher, K. Effect of timing, rate, and form of N fertilization on nutrition, vigor, yield, and berry yeast assimilable N of Grape. Am. J. Enol. Vitic. 61, 327-336. 2010.
Spayd, SE; Wample, RL; Stevens, RG; Evans, RG; and Kawakami, AK. Nitrogen fertilization of White Riesling in Washington: Effects on petiole nutrient concentration, yield, yield components, and vegetative growth. Am. J. Enol. Vitic. 44, 378-386. 1993.
Spayd, SE; Nagel, CW; Edwards, CG. Yeast growth in Riesling juice as affected by vineyard nitrogen fertilization. Am. J. Enol. Vitic. 46, 49-55. 1995.
Thomas, CS; Gubler, WD; Silacci, MW; Miller, R. Changes in elemental sulfur residues on Pinot Noir and Cabernet Sauvignon grape berries during ripening. Am. J. Enol. Vitic. 44, 205-210. 1993.
Thomas, CS; Boulton, RB; Silacci, MW; Gubler, WD. The effect of elemental sulfur, yeast strain, and fermentation medium on hydrogen sulfide production during fermentation. Am. J. Enol. Vitic. 44, 211-216. 1993.
Ugliano, M; Henschke, PA; Herderich, MJ; Pretorius, IS. Nitrogen management – critical for wine flavor and style. Practical Winery and Vineyard. pp. 6-25. May/June, 2008.
Vos, PJA; Gray, RS. The origin and control of hydrogen sulfide during fermentation of grape must. Am. J. Enol. Vitic. 30, 187-197. 1979.
Webb, AD. Volatile acidity. In The Oxford Companion to Wine, Second Edition. JA, Robinson (ed.). Oxford University Press, 1999.
Have something interesting to say? Consider writing a guest blog article!
To subscribe to the Coffee Shop Blog, send an email to stephanie@lodiwine.com with the subject “blog subscribe.”
To join the Lodi Growers email list, send an email to stephanie@lodiwine.com with the subject “grower email subscribe” or click on “join our email list” to the right.
To receive Lodi Grower news and event promotions by mail, send your contact information to stephanie@lodiwine.com or call 209.367.4727.
For more information on the wines of Lodi, visit the Lodi Winegrape Commission’s consumer website, lodiwine.com.


