SEPTEMBER 2, 2019. BY STAN GRANT, VITICULTURIST.

Figure 1. Fruit acidity is an important grape and wine quality characteristic.
In grape berries, acidity imparts tart or sour taste. Both too little and too much acidity are problems for wineries. Too little acidity results in flat and uninteresting wines, but too much acidity results in excessively sharp wines. An amount of acidity that balances sweet and bitter sensations, and provides a crisp, refreshing quality is optimum (Figure 1). Adequate acidity also stabilizes color and proteins, inhibits the growth of spoilage microbes, and enhances the effectiveness of sulfur dioxide additions to wine. Although winemakers can add acid, receiving grapes with desirable acidity simplifies winemaking and avoids additional product costs.
Acids in Grape Berries
The dominant organic acids in grape berries are malic acid and tartaric acid. Like all acids, these molecules have two parts. One part common to all acids is the hydrogen ion (H+). The second part of acids is an anion. The anions of tartaric acid and malic acid are tartrate and malate, respectively (Figure 2).
There are two commonly used methods to characterize acidity in grapes, juice, and wine: titratable acidity (TA) and pH. TA is the common measure of all hydrogen in grapes and wines. TA also represents organic acids and about half of tartrate and malate salts. TA is closely associated with tartness and sourness in grapes and wine, but not wine stability. In grapes, TA commonly ranges between 5 to 10 g/L tartaric acid equivalents. A desirable acidity level is generally between 6.5 and 8.5 grams per liter.
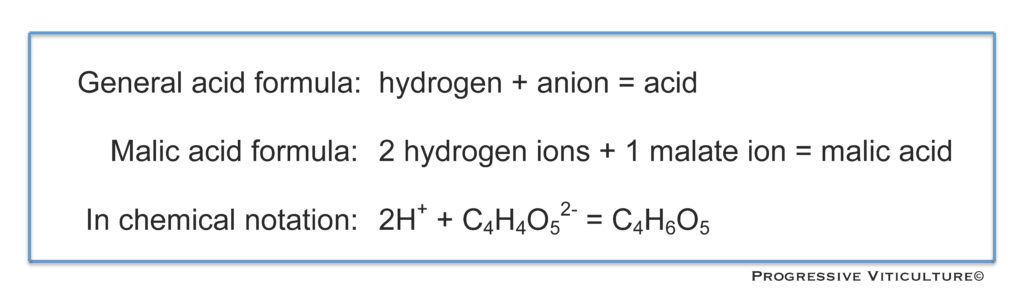
Figure 2. Acid formulas. (Progressive Viticulture©)
pH in Grape Berries
The second common measure of acidity in grapes, juice, and wine is pH. It is the concentration of unassociated or free hydrogen. The acidity measured as pH is considered effective and active acidity. It is important to the chemical and microbial stability of wines, but has little to do with their taste.
Although counter-intuitive, lower values on the pH scale represent greater concentrations of hydrogen in the berry, juice, or wine. As a consequence, pH generally increases as TA decreases. However, berry pH can vary significantly at similar TA levels depending on the ratio of tartaric to malic acid and potassium concentration. Berry pH normally ranges between 2.9 and 4.0. A desirable fruit pH is less than 3.7.
The Seasonal Course of Acidity and pH
Berry acidity is highest during veraison and it diminishes markedly early in the ripening period (Figure 3). Fruit pH, on the other hand, increases during ripening and the rise in fruit pH often correlates with the rise in soluble solids.
There are several reasons for the loss of acidity during fruit maturation: fewer acids are synthesized in the berries, some acids are transformed into sugars and secondary metabolites, acids are diluted as berries grow and increase in volume, positively charged ions other than hydrogen associate with tartrate and malate, and acids are consumed as a source of energy in the process of respiration. pH increases in large part due to the loss of organic acids, but also due to the influx of potassium, particularly late in the ripening period.
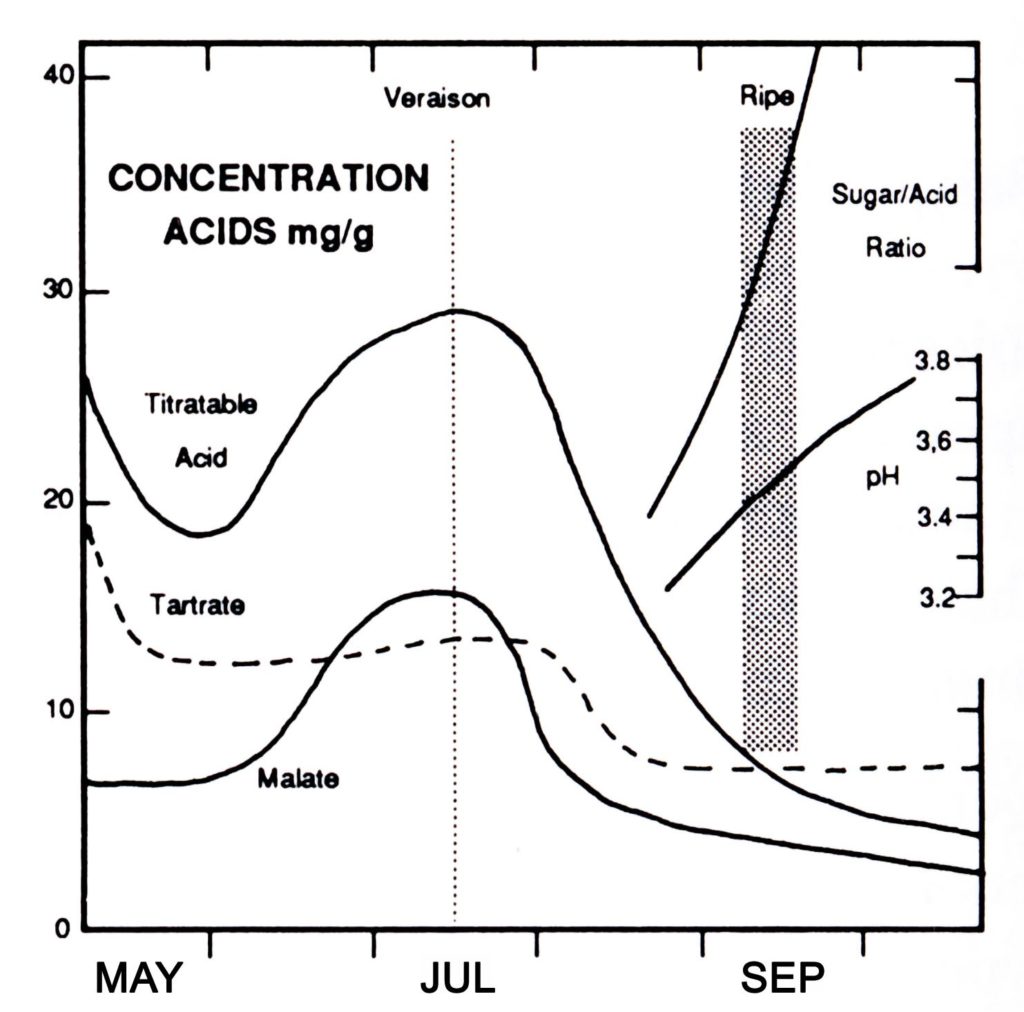
Figure 3. Seasonal changes in berry acidity and pH. (Source: Coombe, 1975 via Coombe & Dry, 1992)
Potassium in Grapevines and Berries
In grapevines, potassium remains unbound and readily moves in vascular tissues, allowing it to serve several unique roles. Affecting the movement of sugar within vines is among them. During ripening, insufficient potassium diminishes sugar transport from leaves and inhibits berry growth, which in turn leads to decreased photosynthesis and increased storage of sugars in leaves. The excess accumulated sugar oftentimes leads to the leaf yellowing (chlorosis) characteristic of potassium deficiency (Figure 4). Even in the absence of visual symptoms, the impacts of potassium deficiency on fruit yield and maturation are detrimental. In addition, insufficient fruit potassium may lead to slow or stuck fermentations at the winery.
Within grape berries, potassium steadily increases as they grow and develop after fruit set. At harvest, berries contain more potassium than any other mineral nutrient, about 5 pounds potassium or more per ton.
Among the functions of potassium in grape berries is neutralization of electrical charge. Like hydrogen, positively charged potassium associates with negatively charged malate and tartrate, which results in potassium malate and potassium tartrate salts. As this occurs, hydrogen leaves the berry, acidity decreases, and pH increases. When potassium accumulation is excessive, fruit pH becomes unacceptably high. Acid addition and ion exchange methods can reduce the pH of grapes delivered to wineries, but they are costly.

Figure 4. Ripening period symptoms of potassium deficiency sometimes appear on younger leaves near the shoot apex. These symptoms include chlorosis and downward leaf curling. (Progressive Viticulture©)
Other Grape Acidity, pH, and Potassium Factors
Climate can impact fruit acidity, especially prevailing nighttime temperatures. Warm night temperatures support a high level of metabolism and metabolism requires energy. As the supply of sugars coming from photosynthesis and storage in the leaves dwindles in the evening, berries use the next most readily consumable sources of energy, which includes organic acids. They are consumed and the energy in their bonds is released during respiration. Malic acid consumption occurs at lower temperatures during respiration than tartaric acid and, as a consequence, it is lost more readily (Figure 3). Malic acid respiration slows late in the ripening period as nighttime temperatures cool and most of the available malate has been consumed.
Some varieties tend to produce ripe fruit with higher acidity than others. Barbara and Colombard are examples of varieties known for high mature fruit acidity. Varieties can also differ in ratios of tartaric to malic acid, which reflect the relative losses of the two acids during ripening. Both Riesling and Semillon tend to have high ratios of tartaric to malic acid. Rootstocks may indirectly influence fruit acidity because they differ in their capacity to take up and translocate potassium.
While there are some localized exceptions, most soils in the Northern Interior of California are low in potassium. Under these conditions, soil potassium commonly has a negative impact on fruit maturation and yield. And while potassium fertilizer applied at excessive rates, such as those used in certain Midwest field trials, can induce high fruit pH, in California application rates are normally moderate to contain fertilizer costs. Consequently, potassium fertilization seldom has a direct influence on fruit pH.
Abundant soil moisture enhances potassium movement within soils, uptake by grapevine roots, and translocation to shoots and the fruit they bear, thereby increasing fruit pH. Probably more important, ample soil moisture is associated with shading within canopies, which promotes premature leaf senescence and potassium movement from senescing leaves to berries. Shading may also slow fruit maturation and the loss of titratable acidity. In contrast, excessive exposure increases berry temperatures and accelerates respirational malic acid loss.

Figure 5. Acidity is especially low and pH especially high in overripe berries. (Progressive Viticulture©)
Conclusions: Vineyard Management and Grape Acidity, pH, and Potassium
The relationships between berry acidity, pH, and potassium are complex and our understanding of the factors affecting them is incomplete. Still, we know some fruit acidity, pH, and potassium factors are beyond our control and we have opportunities to influence others. A vineyard management strategy to positively influence fruit acidity and pH includes the following elements.
First, design vineyards and manage canopies to minimize leaf shading while promoting fruit exposure to dappled sunlight. Second, use potassium fertilizer application timings and rates to meet, but not exceed, grapevine potassium demand. Third, practice regulated deficit irrigation to maintain moderate vine water stress through ripening. Finally, harvest fruit when ripe to avoid the very low acidity and high pH associated with overripeness (Figure 5).
A version of this article was originally published in the Mid Valley Agricultural Services August 2006 newsletter and was updated for this blog post.
Further Reading:
Boulton, R. The relationship between total acidity, titratable acidity, and pH in grape tissue. Vitis. 19, 113-120. 1980.
Boulton, R. The relationship between total acidity, titratable acidity, and pH in wine. Am. J. Enol. Vitic. 31, 76-80. 1980.
Butzke, CE; Boulton, R. Acidity, pH, and potassium for grape growers. Practical Winery and Vineyard. 10-16. Sep/Oct 1997.
Grant, S. Five-step irrigation schedule: promoting fruit quality and vine health. Practical Winery and Vineyard. 21(1): 46-52 and 75. May/June 2000.
Hamilton, RP; Coombe, BG. Harvesting of winegrapes. In Coombe, BG; Dry, PR (ed.). Viticulture. Volume 2 Practices. Winetitles, Adelaide, 1992.
Hale, CR. Relationship between potassium and the malate and tartrate contents of grape berries. Vitis. 16, 9-19. 1977.
Iland, PG. Grape berry ripening: the potassium story. Australian Grapegrower and Winemaker. 22-24. Jan, 1988.
Keller, M. The science of grapevines. Academic Press, Burlington, MA. 2010.
Krstic, M; Moulds, G; Panagiotopoulos, B; West, S. Growing quality grapes to winery specifications: quality measurements and management options for grape growers. Adelaide, Wine Titles. 2003.
Kudo, M; Vagloli, P; Bisson, L. Imbalance of pH and potassium concentration as a cause of stuck fermentations. Am. J. Enol. Vitic. 49, 295-301. 1998.
Morris, JR; Cawthon, DL; Fleming, JW. Effects of high rates of potassium fertilization on raw product quality and changes in pH and acidity during storage of Concord grape juice. Am. J. Enol. Vitic. 31, 323-328. 1980.
Morris, JR; Sims, CA; Cawthon, DL. Effects of excessive potassium levels on pH, acidity, and color of fresh and stored grape juice. Am. J. Enol. Vitic. 34, 35-39. 1983.
Smart, R. and M. Robinson. Sunlight into wine: A Handbook for Winegrape Canopy Management. Winetitles, Adelaide. 1991.
Have something interesting to say? Consider writing a guest blog article!
To subscribe to the Coffee Shop Blog, send an email to stephanie@lodiwine.com with the subject “blog subscribe.”
To join the Lodi Growers email list, send an email to stephanie@lodiwine.com with the subject “grower email subscribe.”
To receive Lodi Grower news and event promotions by mail, send your contact information to stephanie@lodiwine.com or call 209.367.4727.
For more information on the wines of Lodi, visit the Lodi Winegrape Commission’s consumer website, lodiwine.com.


